I want to discuss some science today. Stick with me through this and I may change your world, at least the one your high school science teacher told you existed.
Most recent lie:
Earlier this week I taught Bernoilli's Principle to my physics class. This is a very famous principle dealing with fast moving fluids in a closed system. It is the reason that we get lift using laminar flow over airplane wings, or how your carburetor works to draw fuel in your car engine.
There is currently a heated debate on whether this can be used to explain why a curve ball moves, or my golf ball turns left after it leaves my club.
Technically these involve another principle that is very similar to Bernoilli (Magnus effect), but differing in that they do not require a closed system. The differences in the physics are so minor, that most teachers tell their students they are the same thing. I know they are different, but since we didn't really differentiate between what an open and closed system was (I didn't have time to add a second principle) I told my students a lie.
I am sorry for that, but it is by no means my biggest lie.
Most Blatant Lie:
Electricity is a difficult thing for students to understand. We simplify it down to moving charge through wires and components of a circuit. Students do lab activities with Christmas lights, measuring currents through resistors, and learn about switches and capacitors. I tell them that the lights in their house work under a similar principle as what they are learning in class. Blatant lie.
The lights in their house as with much of what is plugged in uses AC current instead of the DC that we are using in class. Students imagine electrons whipping through the lights, exciting gas molecules or heating tungsten to light their room. I mention briefly the difference between drift current and the electric field, but in the two weeks that I have devoted to electricity, I can't go into all that.
Most Outrageous Lie:
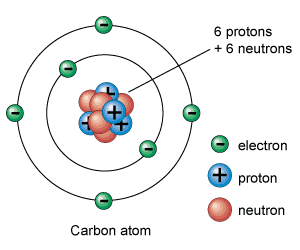
I save my best fallacies for chemistry class. We tell them that everything is made of atoms, and that atoms are made of protons and neutrons. The gross omission is that we stop there. Sure in my advanced class, I cover quarks and leptons and the force carriers, but a general chemistry student is simply not ready for that.
If that isn't bad enough, think about the lie we are spilling when we draw these particles on the board.
This is nothing like what an atom looks like! First off, representing the particles as small spheres is a misrepresentation of what they truly are. What is an electron?.... Its a quantized amount of energy that has a charge of -1.6E-19 Coulombs. It has mass due to its interaction with the Higg's Field giving it a rest mass of 9.11E-34 kg, which changes as it moves. Does an electron have a volume? I have never seen that figure. We know it is localized, but to draw it as a small sphere is completely misleading. The nucleus is worse! It is drawn as a jumble of protons and neutrons, vibrating around each other. Technically is should be a soup of quarks waving in and around each other. Do quarks have a volume? Hard to tell.
Chemistry teachers know that electrons don't orbit like planets as they do in the previous picture. Electrons exist somewhere in orbitals (which for all we know are just mathematical probabilities) that surround the nucleus. In fact, they appear to exist everywhere at once. Why do we draw them as planetary orbits? Well there is a reason. That model explains spectroscopy very well. Students can visualize electrons jumping from one orbital to another. This was worked out a century ago by Neils Bohr. This was his model... 100 years ago!
The scale of that picture of an atom is also completely off. Electrons have 1/2000th the mass of a proton, and the nucleus is WAY smaller than that. As a matter of fact, most of the volume of an atom is empty space. Try discussing the real scale of an atom with students. Since I am made of atoms, and atoms are basically empty space, does that mean that truly I am not here? If I am basically empty space, and the desk is empty space, why can I not pass my hand through the desk? What does it mean to feel something?
Objects are tangible because that empty space (electron orbits mostly) are filled with a few electrons. That makes them negatively charged. The negative empty space in the desk repels the empty space in my hand and thus I can not put my hand through it. In fact, the reason you can see your own hand is the interaction of photons from the lights with that empty space, exciting atoms until they release photons that you can see.
Never lie when it sets up a misconception.
Many would argue that any of the above lies would set up some misconceptions for a student's future understanding of these principles. Most of my hedging of the truth is due to the speed at which I have to cover material. I am very careful to mention many of the above aspects of nature at some point in the year so as to clue them in on what comes next, but many times it is cursory.
We need to be very careful about setting up misconceptions in students. As the above conversation notes, I am not alien to this idea, but there are things that I have seen that concern me. Elementary text books need to be VERY closely scrutinized. I understand not telling a 3rd grader the whole truth about an atom (I don't even stress this to my high schoolers). What I can not overlook is when my son's textbook tells him that mass is anything that takes up space. This is setting up a harsh misconception between mass and volume. What compounds this is the fact that many elementary teachers are not confident enough in the science to know the differences. They rely on that book for their information and may not have the concept separated in their head. This may be a harsh example, but it happens more often than I ever imagined.
What lies do you tell, or what have you seen?
Chris





I never, ever, EVER tell students the real deadlines for yearbooks. Never. In this case, what they don't know won't hurt them!
ReplyDelete